Boy exerting effort and energy to break a stick.
Stable molecules owe their existence to covalent and polar covalent bonds that hold their atoms in place. The strength of these bonds are determined by the amount of energy required to . . . the energy needed to completely separate the bonded atoms.. Just as the breaking of a stick requires the input (addition) of energy, the breaking of a bond is also endothermic (+ΔH). The energy required to break a bond in a gaseous molecule is called the bond energy or the bond dissociation energy and typically reported in units of kJ/mol. Of course the opposite action (unbreaking a stick or making a bond) would be exothermic
Let's calculate the change in bond energy when Hydrogen reacts with Oxygen to form water. First, write the reactants and products showing all the bonding electrons as single, double or triple bonds:
2 H
H + O
O
 2 H
2 HO
H
Then, use this table to look up the bond dissociation energies (BDE) of bonds that are broken and made in the reaction. Notice that all the values in the table are positive - they represent the endothermic process of breaking a bond. However, the bonds on the product side of a reaction are being made, not broken . . . . this fact provides two approaches to calculating the enthalpy (ΔH) of a reaction.
- Use a formula:
ΔH =ΣBDEbonds broken –ΣBDEbonds formed
ΔH =[((2 mol H2 × 1 mol H-H
1 mol H2 × 436 kJ
1 mol H-H ) + (1 mol O2 × 1 mol O=O
1 mol O2 × 495 kJ
1 mol O=O ))–(2 mol H2O × 2 mol H-O
1 mol H2O × 463 kJ
1 mol H-O)]
ΔH = – 485 kJ/mol - Make the BDE of the bonds on the product side negative and add all the Energies.
Bonds broken have a +ΔH . . . . bonds made have a –ΔH
ΔH =(2 mol H2 × 1 mol H-H
1 mol H2 × + 436 kJ
1 mol H-H)+(1 mol O2 × 1 mol O=O
1 mol O2 × + 495 kJ
1 mol O=O)+(2 mol H2O × 2 mol H-O
1 mol H2O × – 463 kJ
1 mol H-O)
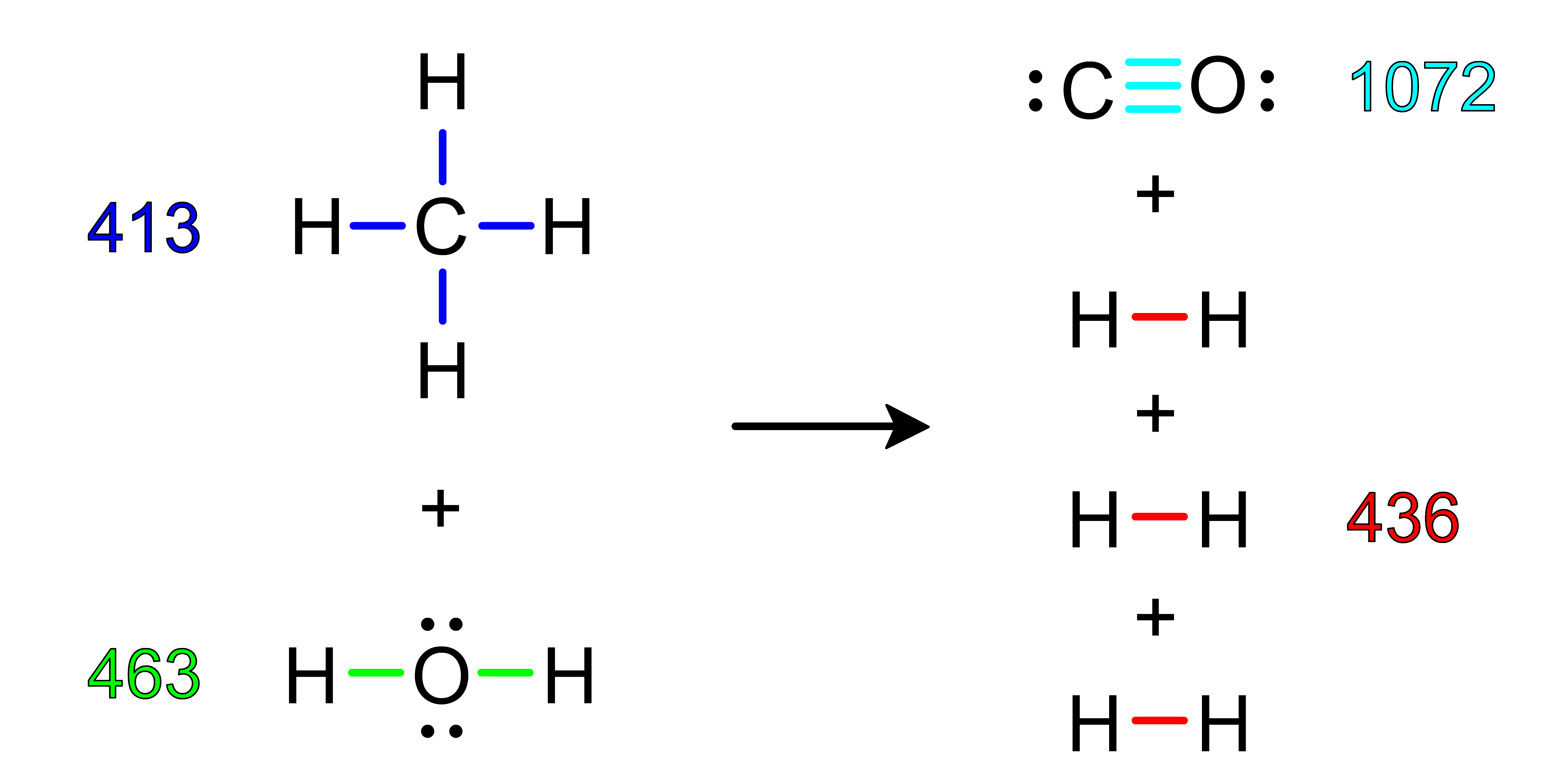
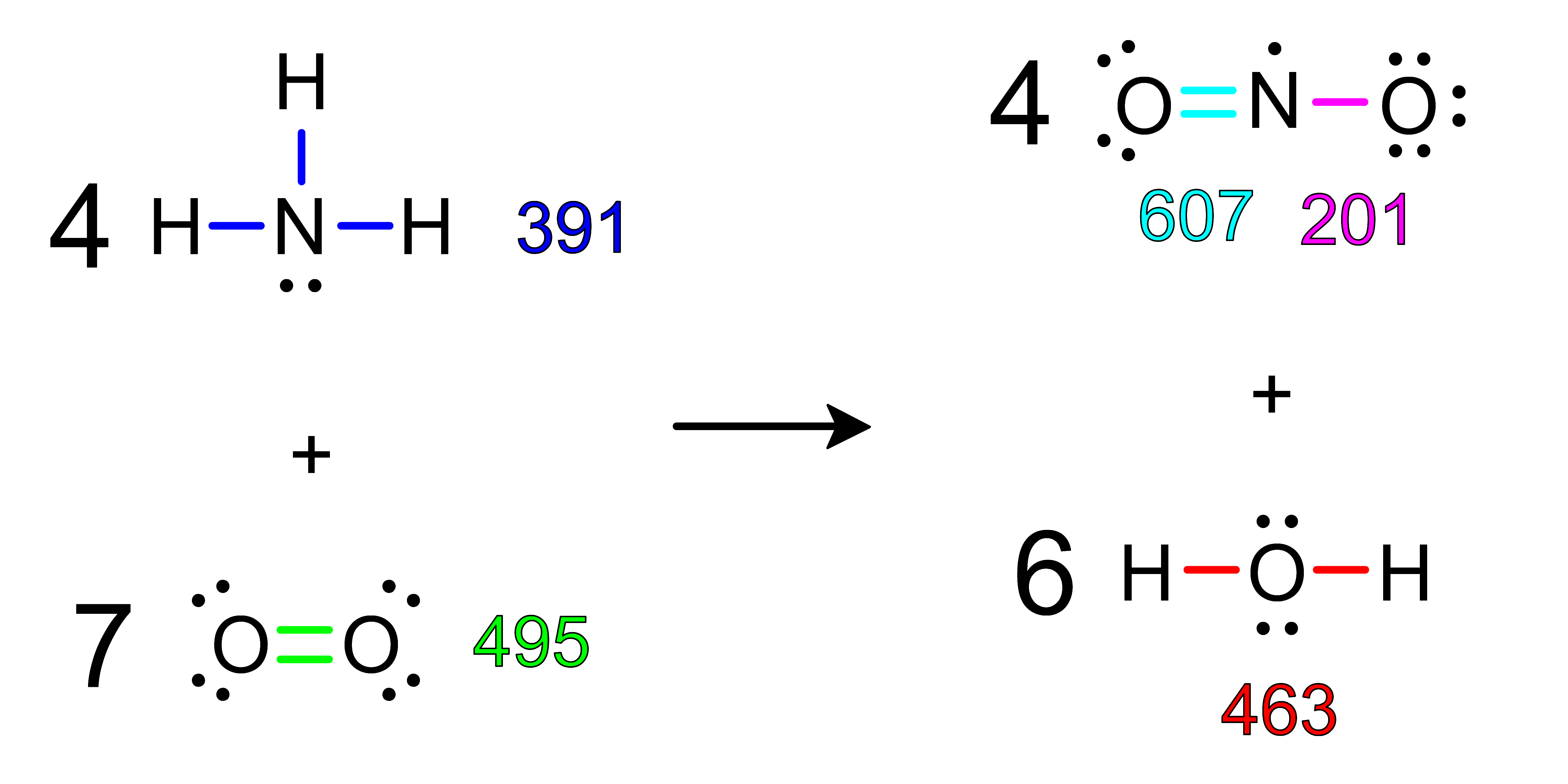
Activity: use the Bond Dissociation Energy Table to calculate the approximate ΔH of the following reactions. After you have determined the answer, click Show Answer to check your work.
Show Answer
Show Answer
Activity: complete the HW 7.5: Bond Enthalpy assignment. Use the Bond Enthalpy Table on the Reference Materials webpage to estimate the ΔHreaction.