Quantitative analysis - is a laboratory method focusing on precise measurements to determine the amount of a substance present in a sample or formed in a chemical reaction. In chemical reactions, the substance that is "measured" can be the focus of the analysis or its value can be used to calculate the quantity of a different reactant or product since a balanced equation connects all substances in the reaction. The . . . . solid, liquid, gas or aqueous of the "measured" substance typically determines the type of quantitative analysis:
- Titration - the reactants and products are soluble in water and form an aqueous solution. Since no change in state occurs during the reaction, the end of the reaction is signaled by the color change of an indicator.
- Gravimetric - a reaction product has a different state of matter than the reactants and the other products.
- solid - if a reaction product is an insoluble salt, the reaction mixture is . . . . passing a heterogeneous solution through a membrane that allow liquids to pass through but not solids., dried and weighed. The solid's mass can be converted to moles which then provides a path to the moles of the other reactants and products using the coefficient ratios in the balanced equation.
- gas - if a reaction product is a gas, subtracting the final mass of the reaction mixture from the intial mass of the reaction mixture gives the mass of the gas. The gas' mass can be converted to moles which then provides a path to the moles of the other reactants and products using the coefficient ratios in the balanced equation.
Titration - an analysis that uses a buret to add the titrant to a solution containing the analyte. A buret is a graduated glass tube, fitted with a stopcock, that dispenses the titrant by controlled rotation of the stopcock. To obtain accurate measurements, you must "prepare" the buret.
- Fill the buret with titrant above the 0.0 mL mark (already completed). Note that the glass tip below the stopcock contains only air - no titrant.
- Move the waste beaker under the buret and click the stopcock 10 - 15 times until a drop of liquid falls into the beaker.
- Now the buret tip has both air bubbles and titrant. Remove the air bubbles by opening the stopcock fully (click/drag) until the bubbles have exited - they won't exit by clicking the stopcock and dispensing one drop at a time.
- If the titrant level is between 0.0 mL and 5.0 mL, go to the next step . . . otherwise, click (or click/drag) the stopcock until you have dispensed enough titrant that its level in the buret is between 0.0 mL and 5.0 mL.
- Replace the beaker with the Erlenmeyer flask (Step 1 complete ✓).
- Add the titrant volume shown in Step 2 (see animation). The curved bottom of the titrant should be at the volume you are trying to deliver. Click the Check Button (Step 2 complete ✓).
- If successful, the animation will display Tutorial Complete.
- Click the Play button to view the first image.
- Read the volume at the bottom of the meniscus - zoom in for better accuracy.
- Use the number pad (or your keyboard) to enter the volume of the titrant to the hundreth's place.
- Click the Check Answer button.
- Submit 3 correct answers to complete the tutorial . . . the animation will display Tutorial Complete.
All aqueous solutions have a distinct curvature (. . . . menisci, from Greek, meaning crescent) at its upper surface when placed in a glass buret. The solvent (water) is attracted to the glass and is pulled up the glass causing the curvature in the center of the tube. The four keys to reading a buret accurately are . . .
- understanding the graduation markings between the whole numbers. Is each graduation 0.10 mL or 0.20 mL?
- positioning your eye perpendicular to the bottom of the meniscus.
- reading the buret - the graduation values increase from top to bottom. Make sure your final reading is between the whole number above and below the meniscus.
- estimating the 'hundredths' place - while the smallest graduations represent the 'tenths' place, you must estimate the level of the meniscus to the 'hundreths' place.
- if the meniscus is exactly level with a whole number (i.e. 10), report the value as 10.00 (the "10" line is actually the 10.0 line and your estimate adds the last "0").
- if the meniscus is exactly level with 10.5 mL, report the value as 10.50 mL (your estimate adds the last "0").
- if the meniscus is between 10.5 and 10.6 mL, estimate the hundreth's place and record a value between 10.51 and 10.59 mL.
At the . . . . both the titrant and analyte are the limiting reactant. They both have 0's in the Final row of the table described in Section 4.4 of a titration, the indicator experiences a color change that alerts the experimenter that the titrant has reacted completely with the analyte. In the activity below you will add an indicator (. . . . phenolphthalein is colorless when the pH is < 8. HCl is an acid and has a pH < 7, so initially the HCl-phenolphthalein solution is colorless. Addition of NaOH (base) from the buret increases the pH. When the pH reaches 8, the HCl + NaOH reaction is complete and the solution turns a faint pink color.) that is colorless in acidic solutions and pink in basic solutions. The phenolphthalein is initially added to the colorless acidic (HCl) solution. Then, as base (NaOH) is added from the buret, the colorless solution turns a pink color that quickly dissipates. Near the equivalence point, an increasingly longer amount of time is required for the pink color to dissipate. Eventually, one drop of the NaOH solution cause the solution to remain a very faint pink color 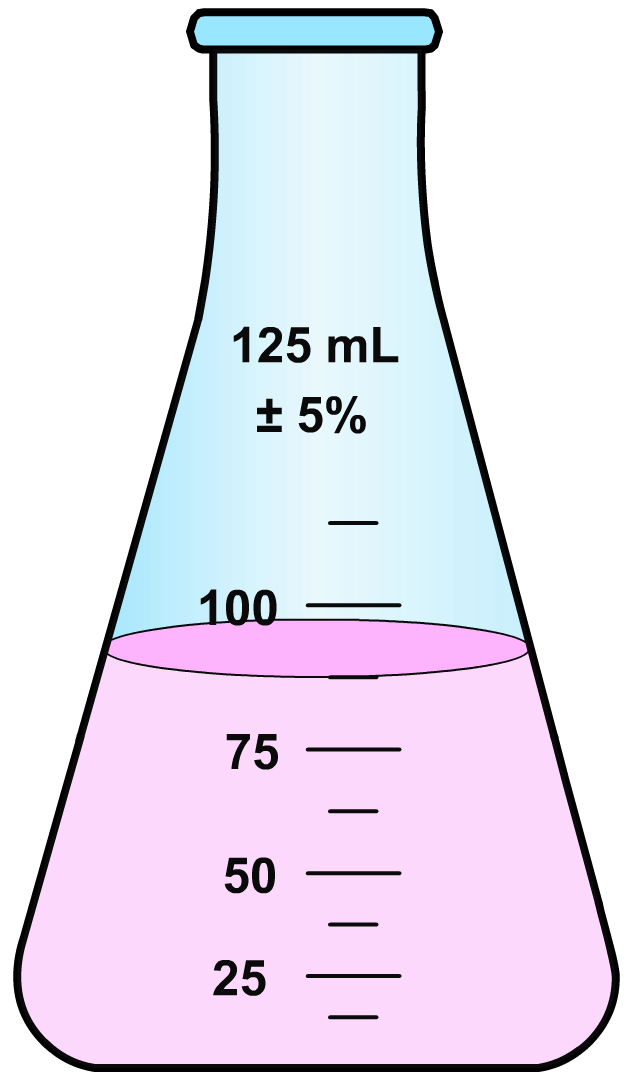 .
.
- Click / drag the phenolphthalein dropper to the Erlenmeyer flask. Mouseup to add 3 drops of the indicator to the HCl solution already present in the Erlenmeyer flask.
- Click / drag the Erlenmeyer flask and HCl / phenolphthalein solution to the ring stand.
- The initial buret reading is mL.
- Move the white card behind the Erlenmeyer flask to enhance your perception of small color changes.
- Rotate the stopcock to add the NaOH from the buret.
- Click to add 1 drop
- Rotate between 10° and 80° to add 1 - 3 drops
- Rotate between 80° and 100° to add a stream of liquid
- Titrations require time and attention to avoid "over-shooting" the endpoint. To speed up this activity, you are given the fact that the buret reading at the equivalence point is mL . Quickly add titrant to within 1 mL of the endpoint. Then add the last milliliter dropwise until one drop causes the solution to turn a permanent light pink color.

Liter solution
Gravimetric - an analysis that measures a change in mass of one component in a mixture. In a typical gravimetric analysis, a sample containing the component of interest is mixed with an aqueous solution forming an insoluble product. This product is isolated by filtration, dried and weighed.
Ag+(aq) + Cl-(aq) → AgCl(s)
If the original sample weighed 0.7011 g and the AgCl(s) weighed 0.9805 g, what is the percent by mass of chloride in the sample?
Step 1: Use the Dimensional Analysis Map 3 to convert the mass of the product, AgCl(s) (0.9805 g), on the left side of the map into the mass of the reactant, Chloride ion, on the right side of the map. Once you have an answer, click Show Answer
Step 2: Use the Step 1 mass and the sample mass to find the percent by mass. Once you have an answer, click Show Answer