Chemists create the ideal reaction environment where mixtures of elements and compounds produce new compounds. In "industrial production facilities", the cost of the desired product is paramount. Among the numerous cost factors are . . . .
- starting materials
- human work hours required to produce the product
- facility cost spent to arrive at a product pure enough to sell
- final amount (mass) of the product
Let's leave the first three for the business owner and focus on the final amount of product. To know if the amount of product formed can be improved upon, the chemist must know the theoretical yield. This is the maximum amount of product that can be formed if the reaction and purification process goes perfectly - reactants are completely converted to products and none of the desired products are lost in the purification process.
As we learned in the last section, the calculation of theoretical yield begins with the limiting reactant.
To find the limiting reactant, pick a product (any product) and calculate how many moles of that product you can make from each reactant.
Use the Dimensional Analysis Map 3 to determine the limiting reagent . . . .
- grams → moles . . . . convert the amount of a reactant (left side of map) into moles (this may take a few steps if the reactant's amount is not given in grams)
- moles → moles . . . . convert the reactant moles into product moles (right side of map). Use the coefficients in the balanced equation as the conversion factor.
- repeat the steps above for each reactant in the chemical equation
The reactant that produces the least number of moles of product is the limiting reactant. The process for finding the limiting reactant also gives the theoretical yield . . . . the moles of product formed from the limiting reactant. The theoretical yield expressed in grams requires an additional conversion using the molar mass (g/mole) of the product.
|
% Yield = |
Experimental Yield Theoretical Yield |
× 100 |
The percent yield is expressed as the ratio of the actual mass and expected mass of a product. At the end of an experiment, a chemist measures the mass of the purified product. This number has very little significance unless the theoretical yield is known and the percent yield can be calculated and reported.
Another common calculation is to determine the remaining mass of an excess reactant after the reaction is complete. This is easily accomplished by setting up the Initial , Change and Final rows creating a table similar to the one used in the previous section. The mass of excess reactant is listed in the Final Grams row. If you don't want to set up a table, you can calculate the mass of an excess reactant using the steps below . . . .
- convert the limiting reactant's (RL) moles ( Change Moles ) into grams of excess reactant (RE) that actually reacted ( Change Grams )
moles RL → moles RE → grams RE - subtract the RE mass that reacted ( Change Grams ) from the RE starting mass ( Initial Grams ) to find the mass remaining at the end of the reaction ( Final Grams ).
RE Starting Mass – RE Mass that reacted = RE Mass in Excess
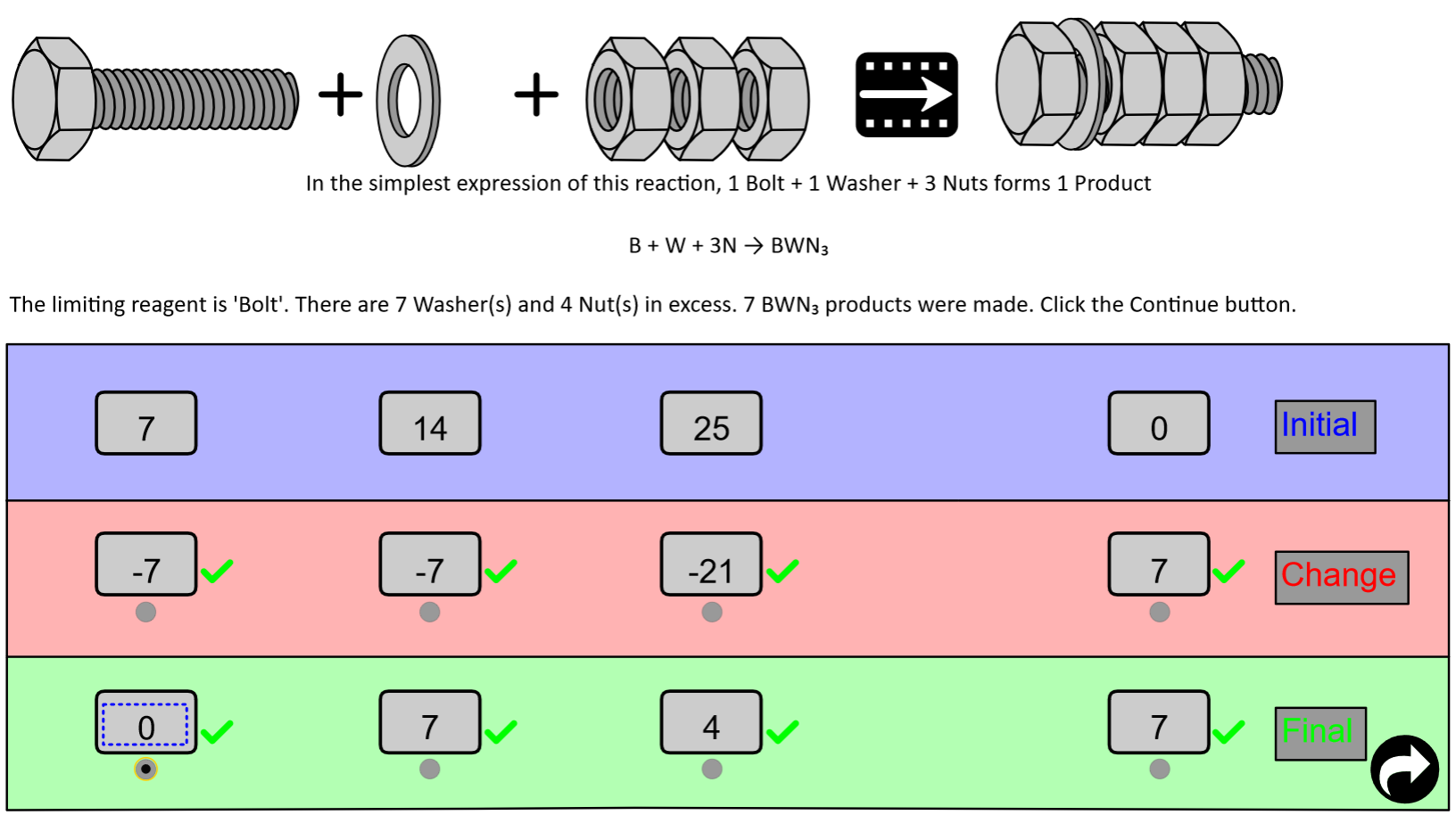
Let's relate theoretical yield, percent yield and excess reactant calculations to the "Nuts and Bolts" tutorial.
- the limiting reactant (bolts) is the reactant that has a "0" in the Final row.
- the theoretical yield is the number of Products formed (7) in the Final row.
- the excess washers are the number of washers (7) in the Final row.
- the excess nuts are the number of nuts (4) in the Final row.
- the percent yield is the actual yield divided by the theoretical yield × 100. Suppose one of the BWN3 products rolled off your workbench and fell into a crack. Now you only have 6 BWN3 products instead of the expected (theoretical) yield (7) from the Final row.
7
Percent Yield = 85.7%
For chemical reactions, there are two Final rows:
- moles Final row.
- grams Final row.
It is common for an excess reactant or a theoretical yield question to specify that the answer must be reported in grams . . . . so, an additional calculation must be made to convert the Final Moles row to Final Grams row using the molar mass (g/mole) of the reactant or product.