
Gilbert Lewis
American chemist
Gilbert N. Lewis (chemist and dean of the college of chemistry at UC Berkeley) made significant contributions in the area of molecular bonding, . . . electrons that reside in the outermost energy level of an atom, and how electron pairing creates molecular bonds. Lewis symbols (atoms) and Lewis structures (molecules) are the typical method for showing valence electrons and chemical bonds. In a few chapters we will learn how Lewis categorized acids and bases on their ability to accept (Lewis acids) or donate (Lewis bases) a pair of electrons.
Lewis Symbols
Lewis symbols present a picture of the valence electrons of atoms and monoatomic ions. In 1902, Lewis began using "cubes" with electrons at the corners to explain to his students how chemical bonds are formed when electrons are transferred. He chose the cube because its 8 vertices provided a physical limitation that reflected the octet rule. The 1902 version of Lewis' symbols for the elements in the second period of the periodic table is . . . .
The series begins with Lithium (1 valence electron) and ends with Neon (8 valence electrons). There can only be 1, 2, 3 or 4 bonding sites because once the number of valence electrons equals or exceeds 5 (N), the valence electrons begin to "pair" which reduces the number of bonding sites. This "cubic" depiction helped Lewis' students visualize why the bonding from Lithium to Neon was 1 (Li), 2 (Be), 3 (B), 4 (C), 3 (N), 2 (O), 1 (F), 0 (Ne).
Over time, the Lewis cubes evolved into the Lewis symbols shown below. Each side of the symbol can "hold" a maximum of two electrons. Sides that only have one electron are "bonding sites" where another atom with an single, unpaired electron can form a bond.
Li• •Be• •B•• •C••• •N•••• ••O•••• ••F••••• ••Ne••••••
Lewis' illustration helps us understand why Lithium oxide is Li2O where each Lithium forms only 1 bond and oxygen forms two bonds . . . . Li •• O•••• •• Li. Because of Lewis' contribution, we understand why
- Li• + •N•••• → Li3N
- •Be• + ••F••••• → BeF2
- •B•• + ••F••••• → BF3
- •C••• + ••O•••• → CO2 (O=C=O)
- •N•••• + ••F••••• → NF3
- ••O•••• + ••F••••• → OF2
- ••F••••• + ••F••••• → F2
- ••Ne•••••• doesn't react
Lewis Structures
Lewis structures show the pairing of electrons between Lewis symbols. The Lewis symbols (reactants) and Lewis structures (product) are used to show the formation of salts (i.e. LiF) and molecules (i.e. BF3) in the reactions below:
Li• + ••F••••• → Li ••F••••••
•B•• + 3 ••F••••• → ••F•••••• B ••F•••••• ••F••••••
It is also common to see bonding electron pairs represented with a bond. The Lewis structure of Lithium fluoride can be written as Li — F•••••• . . . . where a single bond represents 2 electrons.
The Octet Rule
The octet rule is a fundamental bonding principle stating that atoms . . . there are many exceptions to the octet rule but the 2nd period elements Carbon, Nitrogen, Oxygen and Fluorine rigidly adhere to it. to form bonds with other atoms so that both achieve a stable electron configuration consisting of eight valence electrons (an octet). Two atoms attain the octet configuration by sharing, gaining, or losing electrons during bonding. Like the stable nobel gases, atoms surrounded by 8 electrons have a filled s and p electron shell and this culminates in the formation of a stable molecule.
Double and Triple Bonds
Some molecules cannot achieve an octet around each atom with just single bonds. To remedy this instability, adjacent atoms can form multiple bonds. In the examples below, the blue atom counts all the electrons in the blue oval as its octet while the red atom counts all the electrons in the red oval as its octet.
- Oxygen (O2, inhaled as a reactant in mamalian respiration) uses 12 valence electrons to form
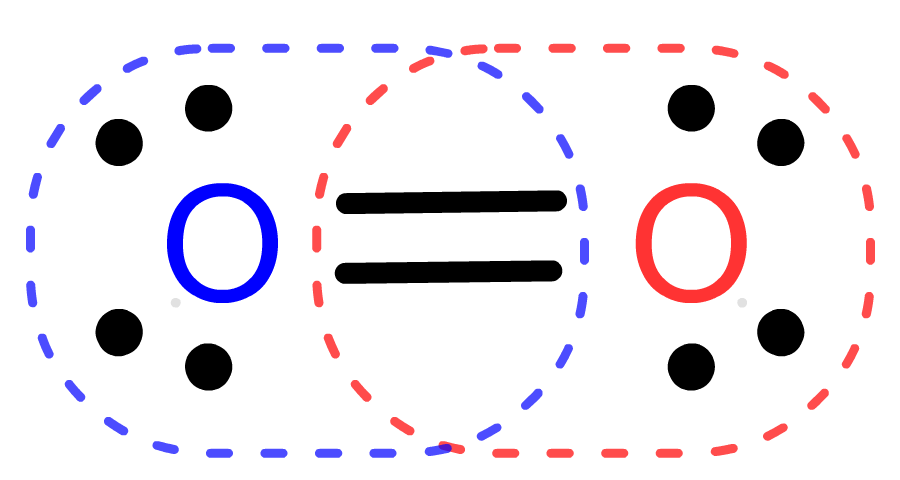
- Carbon dioxide (CO2, exhaled as a biproduct of mamalian respiration) uses 16 valence electrons to form
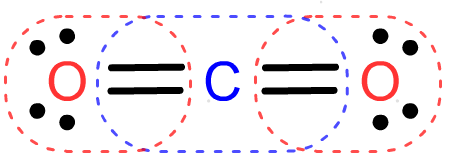
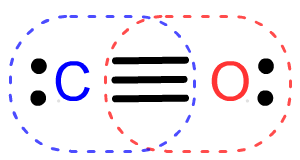
- Carbon monoxide (CO, a gas that stops mamalian respiration at the cellular level) uses 10 valence electrons to form
Writing Lewis Structures - Adherence To The Octet Rule
- Determine the total number of . . . electrons that reside in the outermost energy level of an atom - see Section 6.4 by adding the valence electrons from each element in the formula.
- for anions, add the charge to the total number of electrons
- for cations, subtract the charge from the total number of electrons
- Write the skeletal structure of the molecule
- if the molecule has 2 atoms, write both atoms and connect them with a single bond.
- if the molecule has 3 or more atoms, write the central atom first and connect the terminal atoms with single bonds. Use the Electronegativity Table to the right to determine the central atom.
- Hydrogen is never a central atom - it's not listed in this version of the table for this reason.
- The nonmetal with the lowest electronegativity is the central atom.
- Each single bond used to draw the skeletal structure consists of 2 electrons. Subtract the number of "bonding electrons" from the total number of electrons. Try to satisfy the octets of the terminal atoms by distributing the remaining valence electrons as pairs of nonbonding electrons.
- if there are remaining electrons, place them on the central atom.
- if there are not enough electrons to form an octet on the central atom, convert a non-bonding electron pair on a terminal atom into a double (or triple) bond to increase the number of electrons "owned" by the central atom.
- After you have drawn the Lewis structure, check the following:
- count the electrons used in the structure . . . . make sure they equal the number of electrons from Step 1.
- make sure the non-central atoms with atomic numbers greater than Boron obey the octet rule.
Writing Lewis Structures - Exceptions To The Octet Rule
The central atom in many molecules does not observe the octet rule as described in the following cases:
- Radicals - molecules containing an odd number of valence electrons
- NO has 11 valence electrons . . . it's Lewis structure is ••N•=O•••• . When writing the Lewis structure of radicals, the terminal atoms will obey the octet rule. In a case like NO where there are only two atoms, the most electronegative (Oxygen) element has an octet and the other element has the single, unpaired electron.
- Beryllium, Boron and Carbon - molecules containing Be or B as the central atom will have four or six electrons around the central atom. The additional electron present in the ion BF4– results in an octet of electrons around the Boron. Carbon is the first atom to obtain an octet when found in a molecule . . . . however, the removal of an electron to form a carbocation (i.e. CH3+) results in less than an octet (6 e–) around the central Carbon.
- Hypervalent Molecules - while the 2nd period elements cannot "own" more than 8 electrons (4 bonds), elements in the 3rd and higher periods are larger and can use the space referred to as d orbitals to form more than 4 bonds when they are the central atom.
- PCl5 has 10 e– (5 bonds)
- SF6 has 12 e– (6 bonds)
Activity: use the Lewis Structure interactive to apply the steps above to a variety of molecules and ions.
- Determine the number of valence electrons . . . . move the slider to enter this number.
- Click the Play button to display the central atom, its bonds and the terminal atoms.
- Click the terminal atoms to add electron pairs. Mouseover the central and terminal atoms to view the number of electrons they "own" - bonding electrons are "owned" by both atoms in the bond.
- If all the valence electrons have been added and the central atom does not have an octet, create multiple bonds (click the bond).
- The last two molecules, SF4 and XeF6, are examples where the central atom is hypervalent.
- Submit your answer by clicking the Check button.
Activity: complete the HW 7.3: Drawing Lewis Structures I assignment. Use the LewisDraw interactive to display the Lewis Structure of selected molecules and ions. Once drawn, you can 3-D rotate the Lewis structure to view its geometry.